Cost Of New Covid Nasal Vaccine And How To Book It
Bharat Biotech has developed a new COVID vaccine that will not be injected, instead, it will be inhaled by the patients and will enter the body through Nasal pathways. ₹800 plus taxes is set to be the market per dose cost of this nasal vaccine at private hospitals. Citizens can book their dose on the CoWin portal as well.
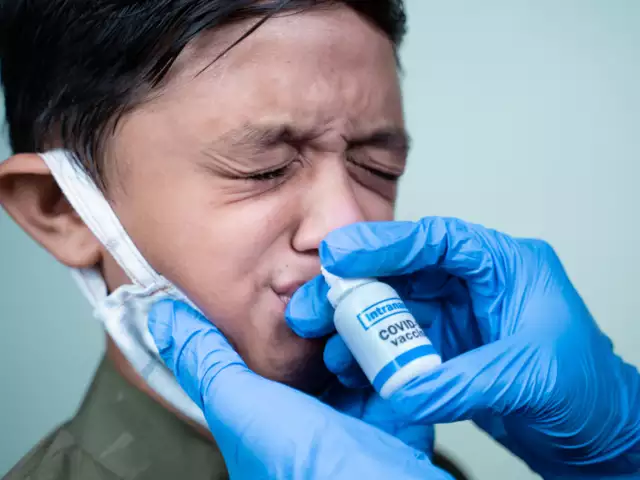
COVID Nasal Vaccine By Bharat Biotech
Named iNCOVACC, this Nasal Vaccine COVID dose will be rolled out at the end of January for all citizens. For bulk procurement, at government centres, this dose will be available at ₹325 per unit.
iNCOVACC is going to be introduced as booster shots for citizens aged above 18 years.
“This is the world’s first intranasal Covid vaccine to receive approval for the primary 2-dose schedule, and as a heterologous booster dose,” said Bharat Biotech.
“The phase-III trials and heterologous trials were conducted for 14 and 9 sites across the country, respectively,” read the statement from Bharat Biotech.
“ Those administered the vaccine demonstrated significant levels of antibody levels in the saliva. Mucosal IgA antibodies in the upper respiratory tract may provide benefit in reducing infections and transmission of the infection,” the Pharma behemoth said during trials.
Bharat Biotech received a green flag, from the central drug regulator, earlier this month, for this Nasal Vaccine as a heterologous booster dose.
“We have achieved the goals we set for ourselves during this pandemic. We have developed COVAXIN and iNCOVACC, two COVID vaccines from two different platforms, with two different delivery systems. The vectored intranasal delivery platform gives us the capability for rapid product development, scale-up, easy and painless immunization during public health emergencies and pandemics,” said Krishna Ella representing Bharat Biotech.
Also read:
Your Old Aadhaar Card Is Required To Be Updated Soon, Says UIDAI
The iNCOVACC was made possible after partnering with major institutions like Washington University and St. Louis, “which had designed and developed the recombinant adenoviral vectored construct and evaluated in preclinical studies for efficacy,” said Bharat Biotech.