ICMR-Backed COVID-19 Vaccine To Launch In June 2021 – Bharat Biotech
After Bharat Biotech received approval from the Drugs Controller General of India to conduct the last phase of the trial of its under-development COVID-19 vaccine, Covaxin, the Hydrebad-based firm has said that the vaccine will be available by June 2021.
As per a report by The Indian Express, the final trial of the Covaxin will be conducted on over 20,000 volunteers across 12-14 states.
“If we get all the approvals in place, I think during Q2 of 2021, we should get the efficacy readout from our phase-3 clinical trial — April, May, June, for example. That is for the full efficacy results,” Sai Prasad, executive director, Bharat Biotech International Ltd told The Indian Express.
Also Read: People Can’t Keep ‘Calm’ Because WhatApps Now Lets You ‘Mute Group Chats Forever’
The Indian Council of Medical Research’s National Institute of Virology vaccine works by injecting the killed version of the COVID-19 virus in the human body to help the human attain immunity.

Besides Covaxin, Serum Institute of India’s ‘Covishield’ is another contender running to launch the COVID vaccine in India. Serum is currently in the process of recruiting volunteers for the third phase of the vaccine trial.
The results of a vaccine’s efficacy show whether it has been able to reduce the number of cases in the inoculated group. “We are committed to doing all our phase-1, phase-2 and phase-3 clinical trials in its entirety, but I think the government may also be considering emergency use approval,” Prasad said.
Also Read: Indians Are Divided After Donald Trump Calls India “Filthy” During Presidential Debate
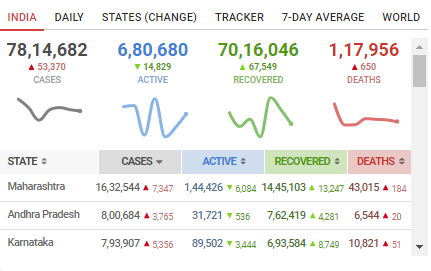
As far as COVID-19 cases in India are concerned, the good news is a continuous decrease in the daily cases for over two weeks now. The Health Ministry has recently said that India has passed the COVID peak back in September and there would be minimal cases left by Feburary 2021.



